8.1 - Introduction to Electrical Charges
8.1.1 - Electrical Charge
History
In the mid 18th century, Benjamin Franklin conducted a series of experiments concerning the attraction between objects and their electrical properties. He would work with materials such as glass rods, silk, and metal, while analyzing their behavior when rubbed against one another. The idea that opposite charges attract and like charges repel was already observed by many, but he tried to uncover the inner workings behind this concept. His idea of positive charges and negative charges went on to become a strong foundation for the field of electromagnetism.

Foundational Concepts
Have you ever rubbed a balloon against your hair and stuck it to a wall? There's no glue or adhesives, so how does it stay up? Based on the title, you can probably guess the reason: electrical charge!
When such materials (like the balloon) act like this, they are considered electrified or electrically charged. When Franklin conducted his experiments, he discovered a set of foundational concepts that govern electrical charges.
- Like charges repel (ex: positive and positive); opposite charges attract (ex: positive and negative)
- You most likely know this one!
- Negative charges are possessed by electrons
- Positive charges are possessed by protons
- Electrical charge is ALWAYS conserved
- You will probably recognize that this is similar to most other laws of conservation in physics.
- In the balloon example, the transfer charge is what makes it electrified. One object gains some negative charge while the other gains an equal amount of positive charge. Remember from mechanics: opposite and equal!
- Electrical charges are quantized
Don't worry if this doesn't make sense right now. We will go over everything in detail below:
Negative and Positive Charges
As you may recall, an atom is made up of electrons (-), protons (+), and neutrons (0, or no charge). Depending on the combinations of electrons, protons and neutrons, the atom is then either positively or negatively charged.
Positively charged objects are at a loss of electrons. Their net charge is positive because there are fewer electrons than there are protons.
Negatively charged objects are at a gain of extra electrons. Their charge is negative because there are fewer protons than there are electrons
Conservation of Electrical Charge
Electrical charge within a closed system isn't created or destroyed. The total net electrical charge will always remain the same, even if the charges move around. The conservation of electrical charge is just like other conservation laws. For example, the conservation of energy, which you learned in mechanics, states that the total net energy in a system remains the same. Energy could be transferred (ex: from potential to kinetic), but there is never any loss or gain. The only exception to this would be external forces like friction. However, the closed system becomes an open system. That is why a loss occurs
It is also important to note that electrons move, but protons don't. That is because the protons are bound extremely tightly to the nucleus, so they are unable to escape. Electrons, however, can float around freely and move.
Let's say that you have two objects: one is positively charged (which we will call object P) and one is negatively charged (which we will call object N). Recall that an object's charge is determined by their electron and proton count. Object P has less electrons (-) than protons (+), so it has a net-positive charge. Object P has more electrons (-) than protons (+), so it has a net-negative charge. The attraction between these two oppositely charged objects occurs because Object N's electrons attract to Object P's net-positive charge.
Quantization of Charge
In 1909, Rober Millikan performed the oil drop experiment. In this experiment, he carefully measured the electrical charge on tiny oil droplets suspended in an electric field. He found that charge is quantized, denoted by the variable , and is always an integral multiple of an amount of charge .
An integral multiple is a number resulting from multiplying an integer with a whole number. In other words, it means a whole number multiplied by another whole number. For example, is an integral multiple of because .
In other words, charges MUST be present in discrete forms, or whole numbers; you cannot have half a charge. Additionally, you cannot have half an electron. It also means that charges exist in "bundles."
Foundationally, this became the formula such that is the total charge, is an integer number, positive or negative, and is the elementary charge.
The idea became that:
- Electrons have a charge of , and negatively charged objects can have charges like and
- Protons have a charge of , and positively charged objects can have charges like and
- Neutrons have a charge of
- There are two types of charges: charges of opposite signs attract and charges of the same sign repel
- Total charge in an isolated system is conserved (conservation of charge)
- Charge is quantized
Ground
When we attach a grounding wire to an object, we are essentially connecting it to a reservoir, like Earth, that can accept and provide electrons freely with negligible effects. Think of a grounding wire like a pipe to the ocean. Because the ocean is massive, any water that we add or remove won't have a noticeable effect on the ocean's overall water amount. Similarly, any electrons which are accepted or provided to Earth (ground) won't affect Earth's overall charge.
Attaching a system to ground allows charges to freely flow in and out of it to reach electrostatic equilibrium. However, this doesn't necessarily mean it will become a neutral charge.
8.1.2 Charging Objects
Conductors and Insulators
- Conductors (e.g. Copper) are materials in which some electrons are free and can move throughout the material.
- Insulators (e.g. Wood) are materials in which all electrons are bound to the atoms and can't move freely at all.
- Semiconductors (e.g. Silicon) have properties similar to both conductors and insulators.
Charging Objects by Friction
Using friction, you can "brush" electrons onto another object. In the video below, you can see how electrons are being transferred between the two objects. However, keep in mind that the net charge of the system did not change. This follows the principles of the conservation of energy. Even though we transferred charge, we didn't add or remove it.
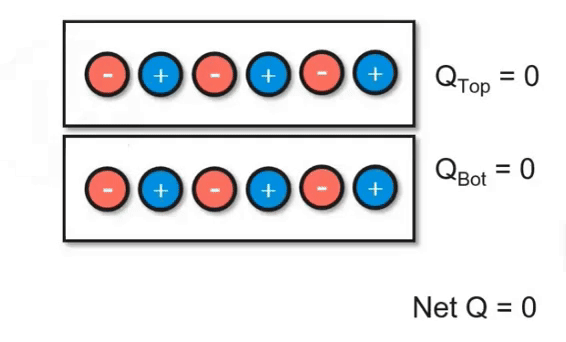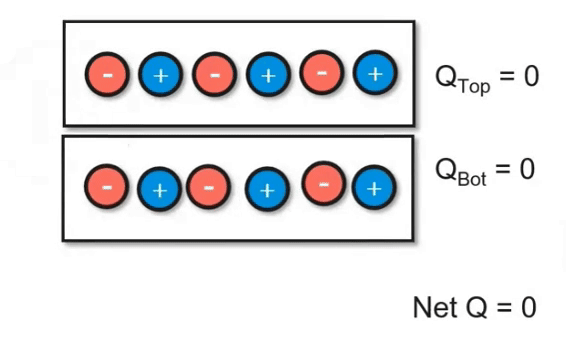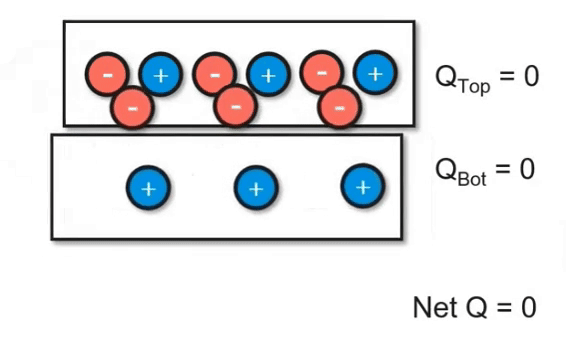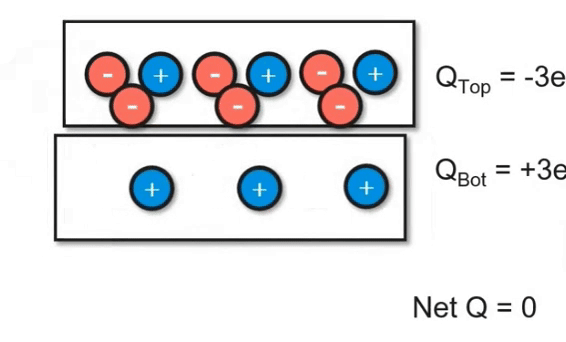
Charging Objects by Conduction
Another way to charge objects is by transferring charge from one item to another to reach equilibrium.
In reality, when two electrical objects undergo charging by conduction, they are actually trying to reach the same electrical potential. We won't go over electrical potential right now though, so we will simply say they are trying to reach equilibrium.
This difference is important because one must remember that objects are not always trying to equalize their charges. For example, a large object and small object with different charges aren't going to automatically equalize; they will still have different charges. For our demonstration, we can assume both objects are the same size and same conducting material.
We start with two metal objects. One has no net charge and one has a positive charge of 2.
We connect both objects with a conductive wire.
In order to achieve equilibrium, an electron will travel to the object on the right.
After the process, both objects have a charge of 1.
Charging Objects by Induction
Induction is the process of charging a conductor, such as a metal, without touching it. Below is an example of how this works:
A conductive metal object, which we will call Object M, starts with a neutral charge (equal electrons and protons).
Object M is brought near another object. However, the second object, which we will call Object C, is charged. Let's say that Object C has a negative charge.
When we bring Object C near Object M, any electrons in M that are close to C will be repelled due to C's negative charge. Remember: since Object M is made up of conductive metal, the electrons can move around freely. Therefore, the electrons will move to the other side of M.
Now, the part of Object M that was closest to Object C will have an overall positive charge since those electrons moved away. However, Object M still won't be charged! The reason is because the electrons haven't left M. They are still there but have just moved away from where Object C is. When we remove Object C, the electrons won't be repelled anymore and the overall charge of Object M won't be different. To make Object M charged, we need to ground it.
The wire connected to the three bars is the symbol for ground.
After we ground Object M, some of the repelled electrons that have moved to the other side will end up escaping through the wire and entering Earth.
When you cut off the ground connection, Object M will have more protons than electrons because those repelled electrons have left. Therefore, the overall charge will be positive.
We can remove object C. Thus, we have used induction to positively charge Object M!
8.1.3 Polarization
Let's say that you rub a balloon against your hair. You probably know that when you lift the balloon, your hair will lift up too. We know this because the balloon has been negatively charged via friction while the hair has been positively charged. This creates an attractive force, so the hair is pulled up.
However, what happens when we put the balloon near some small pieces of paper? The papers jump up and stick to the balloon! But how could this be? The paper was never charged to begin with, and it started off neutral. The answer: polarization.
Remember how the process of charging an object with induction worked? When we brought the square object (net negative charge) near the circular one (net neutral), the electrons within the circle moved away from the square. This resulted in one side having a more negative charge and one side having a more positive charge. Remember, the overall net charge remained the same. All that changes was the charge level on each side of the object.
Similarly, when we hold the negatively charged balloon to the neutral paper, the negative charges within the paper's atoms will move away from the balloon because they are repelled, and the positive charges will move closer to the balloon because they are attracted. This results in the paper atoms having one side that is more negative and one side that is more positive. That is called polarization. When the paper is polarized, it will stick to the balloon because the positive charge wants to touch the negative balloon.
The force of electrical attraction is similar to gravity, becoming stronger when the distance between two charged objects is shorter. That is why the paper sticks to the balloon: the forces of attraction between the positive charges of the paper atoms and the negative charges of the balloon are stronger.
If that didn't make sense, don't worry! What we just talked about is a little out of our scope for this lesson. It will be covered in detail in later lessons.
Useful Resources
8.1.1
- Quantization of charge: the meaning, equation and conservation of charges
- Professor Dave Explains video on charge
- The latter part of the video talks about fields. You don't need to worry about that for now because we cover that in a later section, although you're welcome to learn about it and get a headstart!.
- Crash Course video about charge
8.1.2
- Charging via conduction
- Charging via friction (also called triboelectric charging)
- Charging by induction
- Charging by induction video
8.1.3










